
Cardiovascular heart disease (CVD) is one of the leading causes of death worldwide, with an estimated 17.9 million deaths recorded annually.
With the advancement of technology, new approaches to treating CVDs have been developed to help reduce complications in patients and extend their life span.
Since 2010, UWI St Augustine Chemical Engineering lecturer Dr Shelly Singh-Gryzbon has used fundamental science and engineering principles to conduct research on CVDs and its treatment.
By combining engineering tools like computational fluid dynamics with medical imaging modalities like Magnetic Resonance Imaging (MRI), and computerised tomography (CT), she has been able to develop computational models. These models can then be used to understand and predict the development and progression of CVDs, knowledge that can inform the design and optimisation of medical devices, as well as for pre-procedural or surgical planning.
She told UWI TODAY, “CVDs have affected so many people close to me, and this work provides me with an opportunity to use my chemical engineering background to improve the quality of life of those affected.”
In 2019, Singh-Gryzbon graduated with an undergraduate degree in Chemical and Process Engineering from The UWI St Augustine campus.
However, her interest in CVD research started during her Master’s in Advanced Chemical Engineering at Imperial College London, where she did a course called Transport in Biological Systems.
While fundamental engineering principles can be used to understand the flow of fluids in common mediums like a pipe, similar principles can be used to understand the flow of blood through vessels in the human body.
Dr Singh-Gryzbon developed her first computational model in 2010 for her master's thesis, which looked at modelling the biomechanical stress in the ascending aorta of patients with bicuspid aortic valves.
While doing her PhD in Chemical Engineering, she continued CVD focused research. Her thesis focused on the computational analysis of blood flow and stress patterns in the aorta of patients with Marfan syndrome. After completing her doctorate, she did a postdoctoral fellowship in the Cardiovascular Fluid Mechanics Laboratory at the Department of Biomedical Engineering of the Georgia Institute of Technology in Atlanta.
During the fellowship, she started developing computational models to understand heart valve disease and the devices used to treat these diseases.
Since returning home, she says, “I’m motivated to use my engineering knowledge to improve or design medical devices that are affordable and accessible to our local communities.”
In collaboration with healthcare professionals, mathematicians, and fellow engineers, Singh-Gryzbon is currently developing computational models to investigate potential adverse outcomes associated with heart valve replacement procedures.
“It’s really a collaborative effort, consisting of multidisciplinary and interdisciplinary research.”
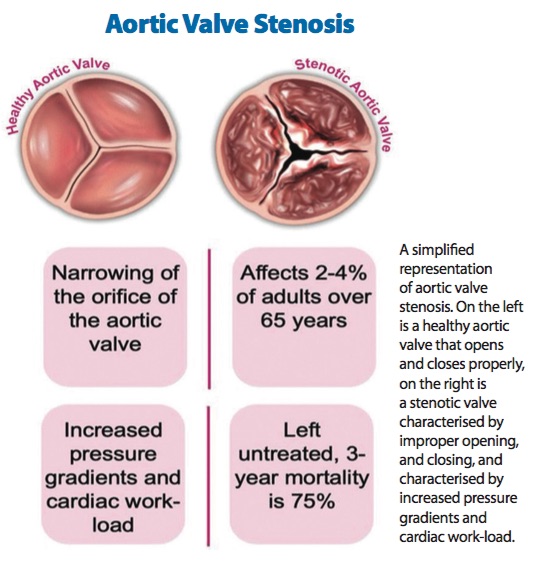
A normal human heart has four valves which open and close to ensure unidirectional blood flow. Sometimes, one or more of these valves may have a defect that hinders its ability to open or close properly, resulting in increased pressure gradients. In these cases, the heart must then work harder to overcome these increased pressure gradients, and if left untreated, they lead to high mortality rates.
Thus, heart valve replacement procedures are used to treat these patients, and involves replacing the patient’s defective valve with a prosthetic valve. These prosthetic valves can be implanted using surgical or transcatheter (non-invasive) approaches.
Thus far, Singh-Gryzbon has made headway into understanding the fluid mechanics associated with transcatheter aortic valve implantation (TAVI) which is used to treat patients with aortic valve stenosis. Aortic valve stenosis is a disease in which the aortic valve does not open properly, and often occurs in older patients due to age-related stiffening.
“The use of [TAVI] was recently expanded from high-risk surgical patients to include intermediate and low-risk surgical patients, raising concerns about the long-term durability of the devices,” she explains.
One adverse outcome of TAVI is the development of thrombosis (blood clots) on the device. Various studies have demonstrated that blood flow patterns, particularly low blood velocities, influence the development of thrombosis on the device.
So, Dr Singh-Gryzbon is using her models to understand how the flow patterns are influenced by the device deployment (or positioning) and device geometry.
It is anticipated that these factors can be optimised to reduce the risk of thrombosis. The goal of her current work is to develop a predictive tool to help clinicians determine the risk of thrombosis in TAVI patients.
“Based on what we’re finding, we can propose alternative design features to medical device manufacturers,” she says. “We can also provide feedback to healthcare professionals on the optimal deployment of the device.”
To help expose her undergraduate students to these engineering applications, Dr Singh-Gryzbon has enlisted some of them to assist with various aspects of her research. She has also introduced some of these applications in her undergraduate teaching through class projects. In doing so, she hopes some are inspired to apply their engineering knowledge to medical applications.
She says, “the future of medicine is ultimately patient-specific healthcare. To get there, we need a host of tools, including computational modelling, which will facilitate the patient-specific planning of procedures.
“It can be challenging, but it is ultimately something which can improve the way in which patients are evaluated, assessed, and treated.”