At the height of the Covid-19 global pandemic, the worldwide demand for medical and personal protective equipment caused countries to compete for scarce supplies. Richer countries outbid poorer ones, driving up prices for test kits and personal protective equipment (PPE). Some also banned exports of vital medical equipment from their countries and seized shipments en route to other ports, leaving developing countries in the lurch.
In early April 2020, Dr Catharina Boehme, the chief executive of Foundation for Innovative New Diagnostics, which collaborates with the World Health Organization (WHO) in helping poorer countries access medical tests, told the New York Times: "There is a war going on behind the scenes, and we’re most worried about poorer countries losing out."
Considering this scramble for resources, The UWI partnered with the Ministry of Health (MoH) and local manufacturers to create alternative sources of PPE and medical equipment that could be produced on demand, locally and affordably. The aim was to design and manufacture safe and effective equipment to supplement current supplies and to prepare for increased demand should the number of Covid-19 cases rise within the region.
According to the MoH, throughout an entire course of treatment for one patient, health professionals use 85 surgical masks, 90 N95 respirators and 30 face shields. These numbers can vary depending on the severity of the cases.
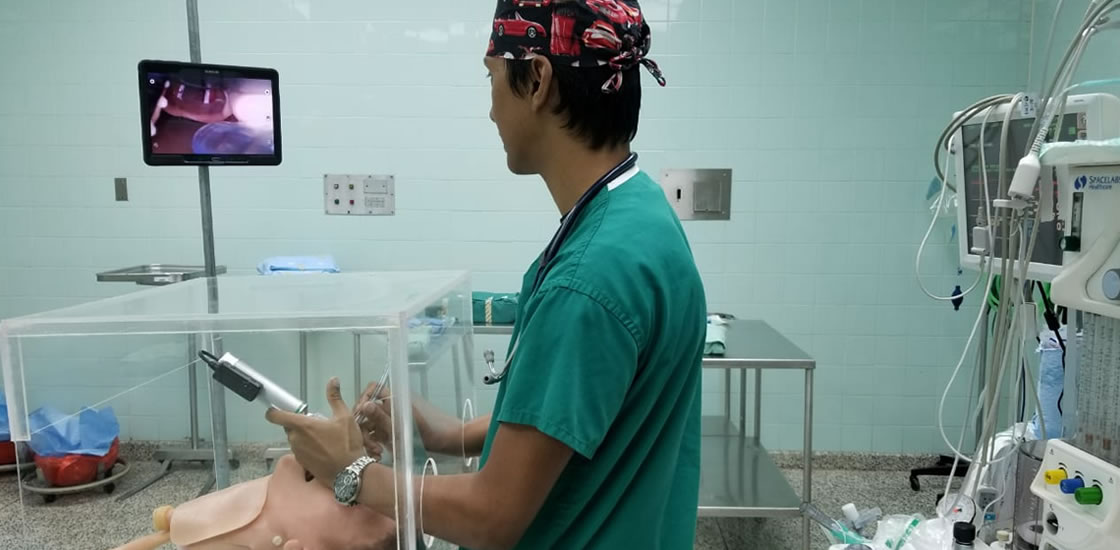
As part of the joint Covid-19 response, The UWI is developing face shields, respirators, face masks, laryngoscopes, aerosolisation hoods, swabs, gowns, slit lamp breath shields and ventilators in response to requests from the MoH and others in the health sector.
The St Augustine Centre for Innovation and Entrepreneurship (STACIE) is coordinating UWI’s collaborative Covid-19 response, including this engineering and manufacturing initiative. The Trinidad and Tobago Manufacturers Association (TTMA) partnered with STACIE to identify local manufacturers who could retool or use their existing production lines to produce the equipment.
Face Shields
The team of UWI engineers and medical experts, in close collaboration with CARIRI, designed, prototyped and developed specifications for face shields for use by local health care professionals. By partnering with the TTMA and Label House Limited, the joint effort has resulted in a donation of 3000 face shields to the MoH. Through a licensing agreement between The UWI and Label House Limited, units have also been produced for sale to the public.


Jeevan Persad, a senior engineering technician in the Department of Electrical and Computer Engineering, leads The UWI team and said it took a few tries before they landed on the right shape and thickness of the face shields. They had to ensure that the shields were safe and comfortable for medical staff to use.
Persad also described how important it was to listen to the doctors who would ultimately use the face shields to land on a final design. The doctors would say whether the shields conformed to their faces or if they hurt or left marks after prolonged use.
Label House executive director, Ryan Lewis, added: “We did around 12 or 13 options ... you had to take into consideration things like the tight environment they’re working in, wires that come across their shoulders. They have to move and [the wires] could get caught [in the face shields].”
Sourcing the plastic for the shields was the next big challenge for the team.
“When we started calling [foreign] suppliers of plastic they were out of stock, or they had pivoted, and they were using plastic to make face shields. So, we decided to look for local stock,” Persad said.
Eventually, they found a local producer who could provide PET (polyethylene terephthalate) from recycled plastic bottles. But while the recycled plastic was fine for products like egg crates that manufacturers were already producing, the quality had to be improved for medical use.
“We worked with them to improve the quality,” Persad said, “and they put more virgin plastic in, which increased the clarity.”
After the team settled on the right combination of plastic of a certain thickness, elastic and sponge, the face shields went into production. The UWI team worked closely with doctors and the MoH to test and quickly get approval for the final product.
For Label House, the collaboration meant that they were able to create a new product line that was tested and certified by The UWI.
“When you go to market with effective research and backing, that reduces the ability of external parties to leapfrog you or take shortcuts,” Lewis said. “I think that’s where you’ll see a difference in quality and just trust in the product that’s being sold.”
Lewis also said that he was excited about the potential for export for T&T manufacturers. “There is a global shortage,” he said, “so if we do the research of where the gaps are ... for what the CARICOM region requires—and also the Central American region— we can supply it. I think there’s a big opportunity.”
Face Masks
 The T&T government has encouraged public use of cloth masks. Persad believes, however, that it isn't always clear how they can be used properly. He said that questions remain about choosing which materials to use to make the masks, how they should fit, how they should be cleaned and when they should be replaced.
The T&T government has encouraged public use of cloth masks. Persad believes, however, that it isn't always clear how they can be used properly. He said that questions remain about choosing which materials to use to make the masks, how they should fit, how they should be cleaned and when they should be replaced.
And that’s why The UWI, in collaboration with Cariri and the MoH, developed guidelines that it has shared with NIPDEC. These guidelines, that were based on several international standards, including ASTM and AFNOR standards, help better inform the public about how to make masks that work. The WHO has since released its own guidelines for face masks, which are aligned with those developed at UWI.
Miguel Andrews, a physicist at Cariri, described how they tested locally available fabric samples to narrow down the best materials for the masks. They tested a parameter called filtration efficiency, which determined the fabric’s ability to filter particles from aerosol.
“The trick is, it must still be breathable as well,” Andrews said. “You want it to filter out particles, but you still want air to pass through. If you have 100% filtration, it’s like a gag.”
Now, manufacturers selling cloth masks can have their products tested at Cariri to determine how well they filter micro-particles. When masks test better than the N75 level (the filtration level of surgical masks), manufacturers can share their information with the TTMA to be listed as certified suppliers. These suppliers can then use the TTMA approved label on their products and packaging so consumers can identify that their products have been tested.
Ventilators
Some officials feared a possible ventilator shortage due to high costs and the difficulty procuring them from overseas. In response to this concern, the team, through consultation with medical professionals, designed the prototype for UWI-Vent—a ventilator that will assist those with acute respiratory distress syndrome (ARDS).
Anticipating 2nd and 3rd waves of the virus, the health ministry set a August 2020 deadline for building the ventilator prototype. Dr Antony Parkinson of the North West Regional Health Authority (NWRHA), who is managing the intensive care unit response across Trinidad and Tobago, headed the team of doctors that advised on the specifications for the ventilator. He is charged with leading subsequent testing of the prototype.
But sourcing foreign parts remained a challenge. The specialised parts needed to build the ventilators were either out of stock or banned from being exported.
“We would find a supplier who had the parts in stock, but after we placed the order, we would get an email cancelling the order,” Persad said. Once suppliers learned the final port of destination for the parts was outside of the US, Persad added, the order would be cancelled.
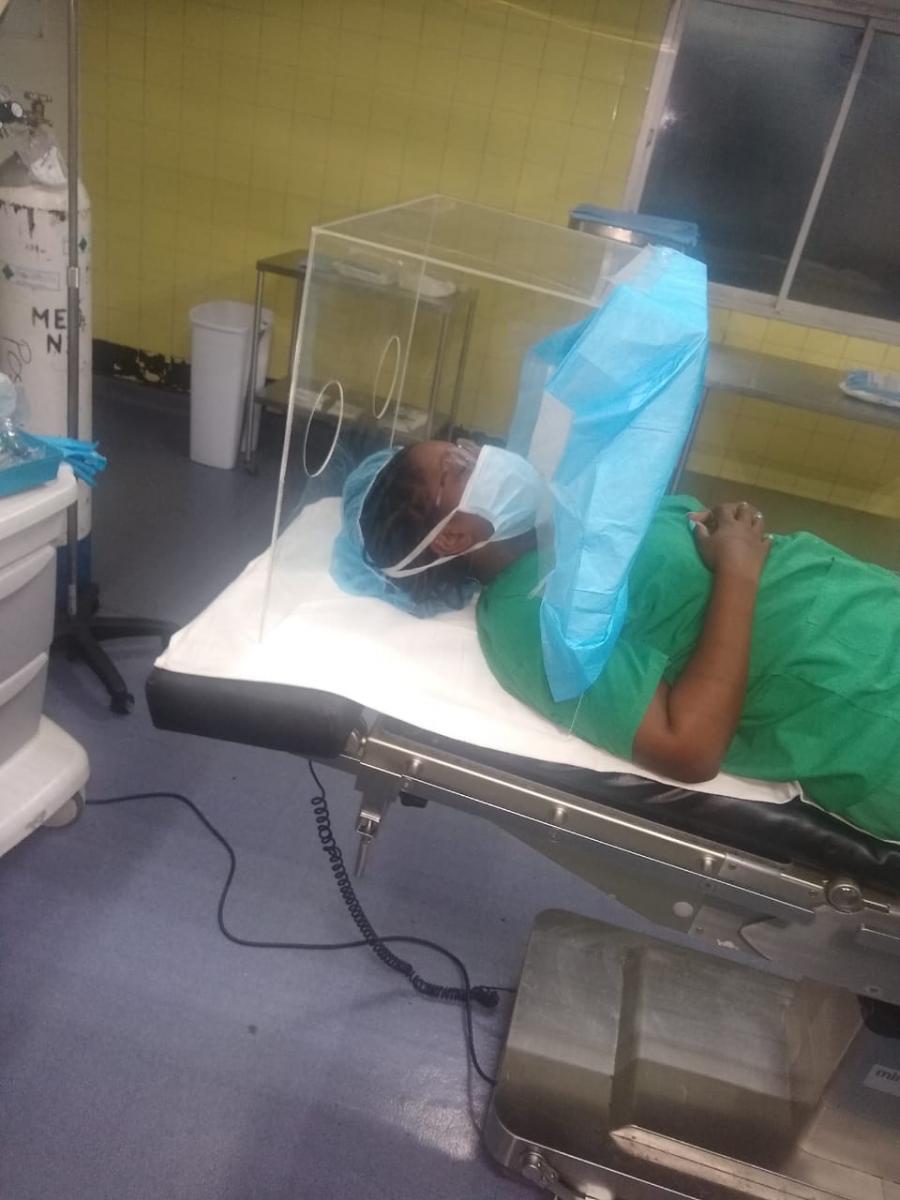
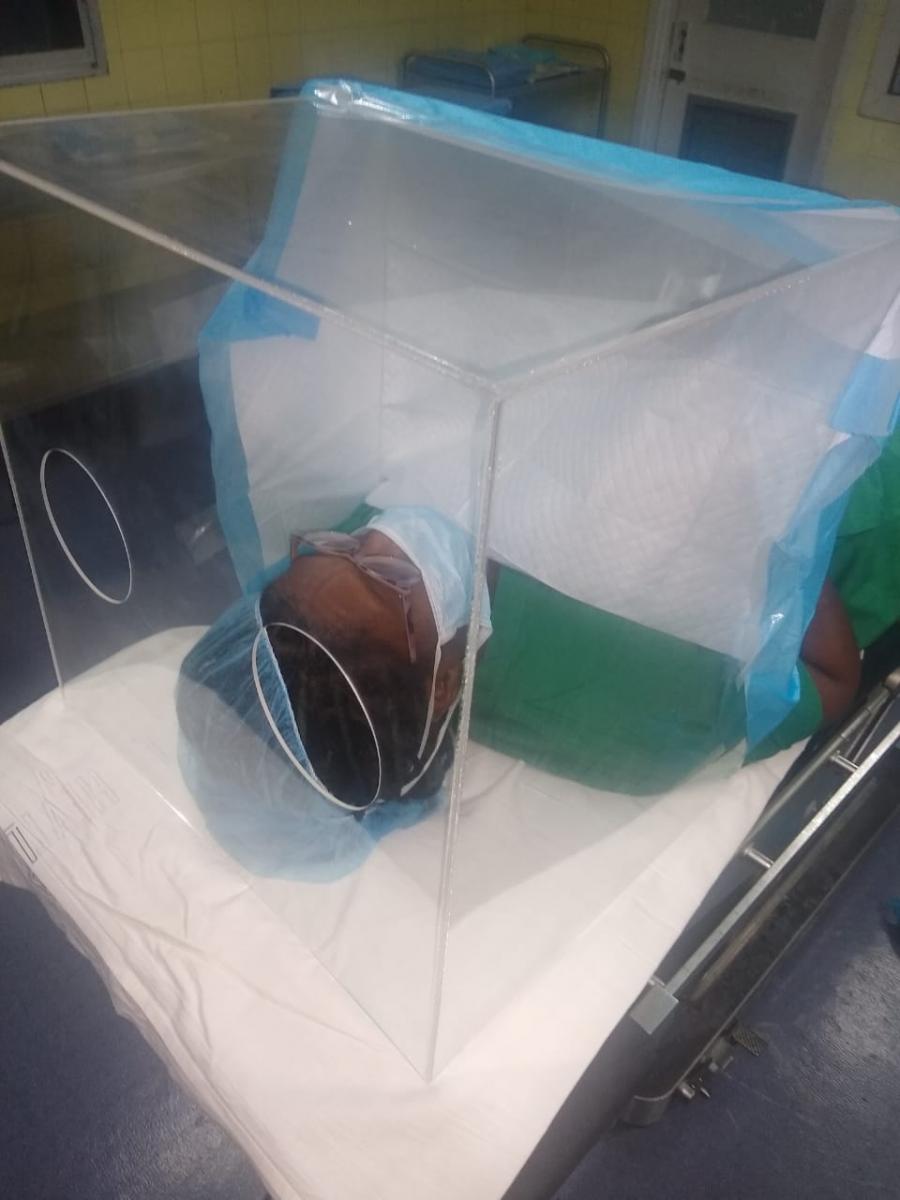
The team needed to find a way around the restriction of exports of medical equipment. That’s when they looked outside medical suppliers.
By leveraging existing industry relationships, STACIE was able to enlist financial, logistical and procurement support to help the team overcome these challenges. Oil and gas sector and food processing industry companies, who already have developed procurement systems, helped source the needed parts. The American Chamber of Commerce (AMCHAM), shipping company eZone Limited, MoH, Republic Bank Limited, and the National Gas Company (NGC) have all provided valuable support to secure the prototype’s bill of materials and necessary testing equipment.
Persad said that once a prototype was made and ventilators went into production, he saw the potential for exports. As the global supply is focused on the US and Europe, Caribbean and Latin America countries are underserved.
“If they know they can get ventilators right here in Trinidad and Tobago – and for much cheaper – this is an opportunity for local manufacturers.”
The work of the team has attracted the attention of the Pan American Health Organization (PAHO), who invited the team to present, alongside teams throughout Latin America who are working on similar ventilator design projects.
N95 Respirators
Work is also ongoing with MIC to develop a mould from The UWI’s 3-D printed prototype that would be used to produce N95 equivalent masks, designed to NIH specifications. Once the mould is produced, a new prototype using the materials intended for final mass production will be made and tested.
To create respirators that could be reused, the team at UWI designed the new masks with a disposable filter that provided N95 equivalent protection. This means that doctors and nurses could keep a single respirator and replace just the filter after use.

As stay at home orders have been lifted, Persad warned we should remain vigilant. He said that it was still important to continue to produce PPE and continue the work the team has started to be prepared in the event of a second or third wave of the virus.
“It might seem like paranoia to have these things if nothing happens,” he said. “But it’s better to be paranoid and be prepared than to not have this equipment if we need it.”








